
CELL IMMORTALIZATION
Primary cells are typically non-immortalized cells that are directly isolated from living tissue and have a limited lifespan in culture. They undergo a finite number of divisions before entering senescence, which makes them less suitable for long-term studies or large-scale experiments. Immortalization of primary cells is a process that allows these cells to bypass normal cellular senescence and proliferate indefinitely, making them more useful for research purposes.
Reasons for immortalizing primary cells:
- Extended Lifespan: Immortalized cells can be cultured for many generations, providing a consistent and renewable source of cells for experiments.
- Consistency: Immortalized cell lines offer a more uniform population of cells, reducing variability between experiments and improving reproducibility.
- Large-Scale Studies: Immortalized cells can be grown in large quantities, which is essential for high-throughput screening, drug discovery, and other large-scale applications.
- Genetic Manipulation: Immortalized cells are often more amenable to genetic modifications, such as CRISPR/Cas9 gene editing, which allows researchers to study gene function and disease mechanisms.
- Disease Modeling: Immortalized cells can be used to create stable cell lines that model specific diseases, enabling researchers to study disease processes and test potential therapies over extended periods.
- Cost-Effectiveness: Maintaining immortalized cell lines is generally more cost-effective than repeatedly isolating primary cells, which can be time-consuming and expensive.
- Reduced Ethical Concerns: Using immortalized cell lines can reduce the need for continuous isolation of primary cells from animals or human donors, addressing some ethical concerns.
- Immortalized cell lines play an essential role in 3D bioprinting technologies as they maintain stable phenotypes and functions over an extended time (Ma et al., 2024).
Methods of Immortalization:
- Viral Oncogenes: Introduction of viral genes (e.g., SV40 T antigen, HPV E6/E7) that disrupt normal cell cycle regulation.
- Telomerase Activation: Overexpression of telomerase (e.g., hTERT) to prevent telomere shortening, a key factor in cellular aging.
- Genetic Engineering: Using CRISPR/Cas9 or other techniques to knock out tumor suppressor genes (e.g., p53) or activate oncogenes.
- Chemical Treatment: Exposure to carcinogens or other chemicals that induce genetic changes leading to immortalization.
While immortalized cells are valuable tools in research, it is important to note that they may not fully replicate the behavior of primary cells or in vivo conditions. Therefore, findings from immortalized cell lines should be validated using primary cells or animal models when possible.
The following list includes some types of cells successfully immortalized by Capital Biosciences:
Human Esophagus smooth muscle cells (hESMC)*
Primary fibroblasts
Skin fibroblasts
FIG4 mutant fibroblasts
Fallopian tube secretory epithelial cells
V922 cells*
Taste cells*
Laryngeal & hypopharyngeal epithelial cells
Primary endometrial epithelial cells
Thyroid cells
Kidney epithelial cells
Lung epithelial cells
Hepatocytes
Peripheral Blood Mononuclear Cells (PBMC)
Placenta cells
Murine Embryonic fibroblasts (MEFs)*
Skin fibroblasts*
Rat Primary valve intersitial cells
Hepatic Sinusoidal Endothelial Cells (Sprague Dawley Rat)
Hepatic stellate cells (Sprague Dawley Rat)
Liver cholangiocytes (Sprague Dawley Rat)
Hepatocytes (Sprague Dawley Rat)
Canine Retinal Pigment Epithelium Cells
Fibroblasts
Bovine Mesenchymal Stem Cells*
Calf Thyroid cells
Swine Macrophages from lung and blood*
Alveolar macrophages
Muscle Skeletal Cells
Ovine Aortic valve interstitial cells
* Several immortalized cell lines were obtained by different methods of immortalization.
Publications:
Tsang, G., Cui, L., Farquharson, C., Corcoran, B. M., Summers, K. M., & Macrae, V. E. (2017). Exploiting novel valve interstitial cell lines to study calcific aortic valve disease. Molecular Medicine Reports, 17(2), 2100. https://doi.org/10.3892/mmr.
Ma, Y., Deng, B., He, R., & Huang, P. (2024). Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication. Heliyon, 10(3), e24593. https://doi.org/10.1016/j.heliyon.2024.e24593
Project example: Immortalization of rat hepatocytes using temperature sensitive SV40 T antigen Ts58A.
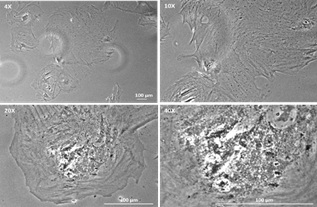
Figure 1. Non-infected Sprague Dawley rat hepatocytes after 2 weeks in culture.
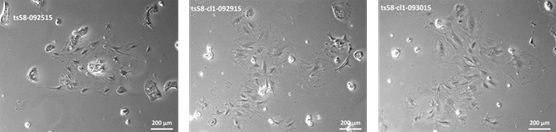
Figure 2. Colony formation in SD Hepatocytes infected with Lenti-SV40-ts58A.
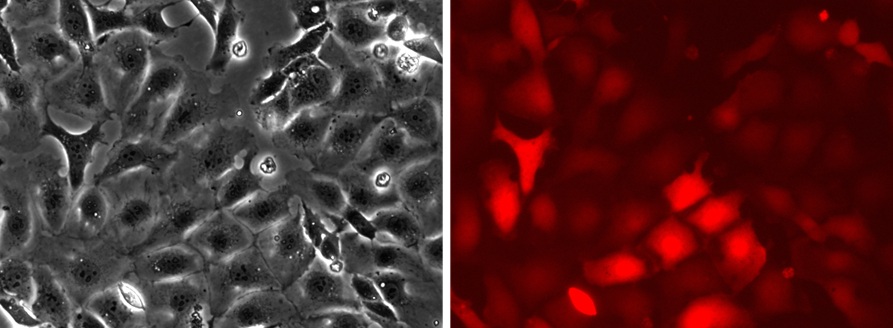
Figure 3. Imortalized SD Hepatocytes transduced with Lenti-RFP.

